CATEGORY
Regulatory
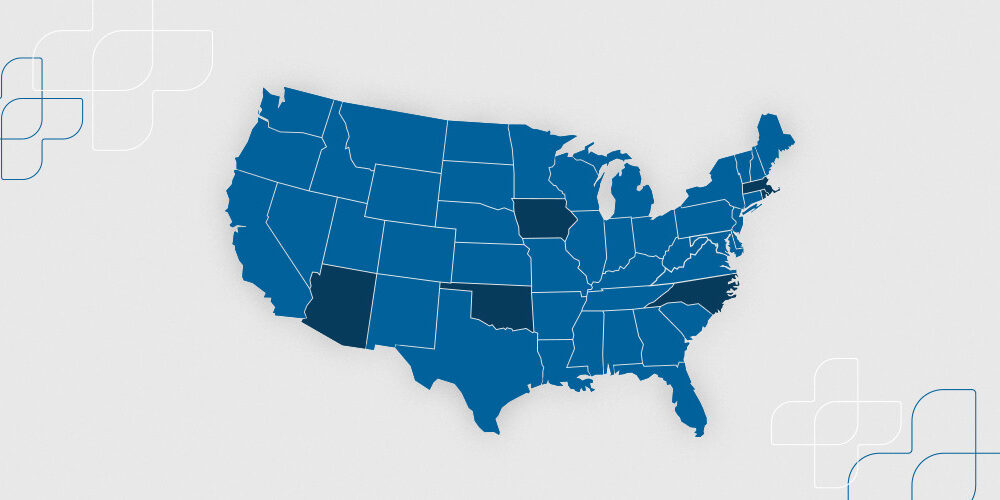
The Next Wave of 2020 EPCS Mandates: What You Need to Know
Effective January 1, 2020, Arizona, Iowa, Massachusetts, North Carolina, Oklahoma, and Rhode Island,...
Pennsylvania Joins the Battle Against Opioid Abuse on October 24, 2019
Electronic prescribing of controlled substances (EPCS) is gaining momentum as an effective weapon...
Four Steps to Comply with EPCS Regulation Deadlines in 2022
Using an EPCS-certified Electronic Prescribing tool is an efficient, secure way to write...
Four Steps to Comply with EPCS Mandates—for Individual Practitioners
Deadlines are around the corner Using an electronic prescription tool is more than...
Four Steps to Comply with EPCS Mandates—for Institutional Practitioners
Deadlines are around the corner Using an electronic prescription tool is more than...
RXNT Applauds Passage of Law to Combat Opioid Crisis
RXNT, a leader in healthcare technology solutions, applauded President Trump’s signing of the...
Opioid Legislation Signed into Law
President Trump signed into law opioid legislation that expands the use of telehealth...
The Benefits of Cloud Solutions in Healthcare
Rapidly advancing technology has changed the game for nearly all industries. The healthcare...
Senate Passes Opioid Crisis Response Act of 2018
The United States Senate passes The Opioid Crisis Response Act of 2018 99-1....